News
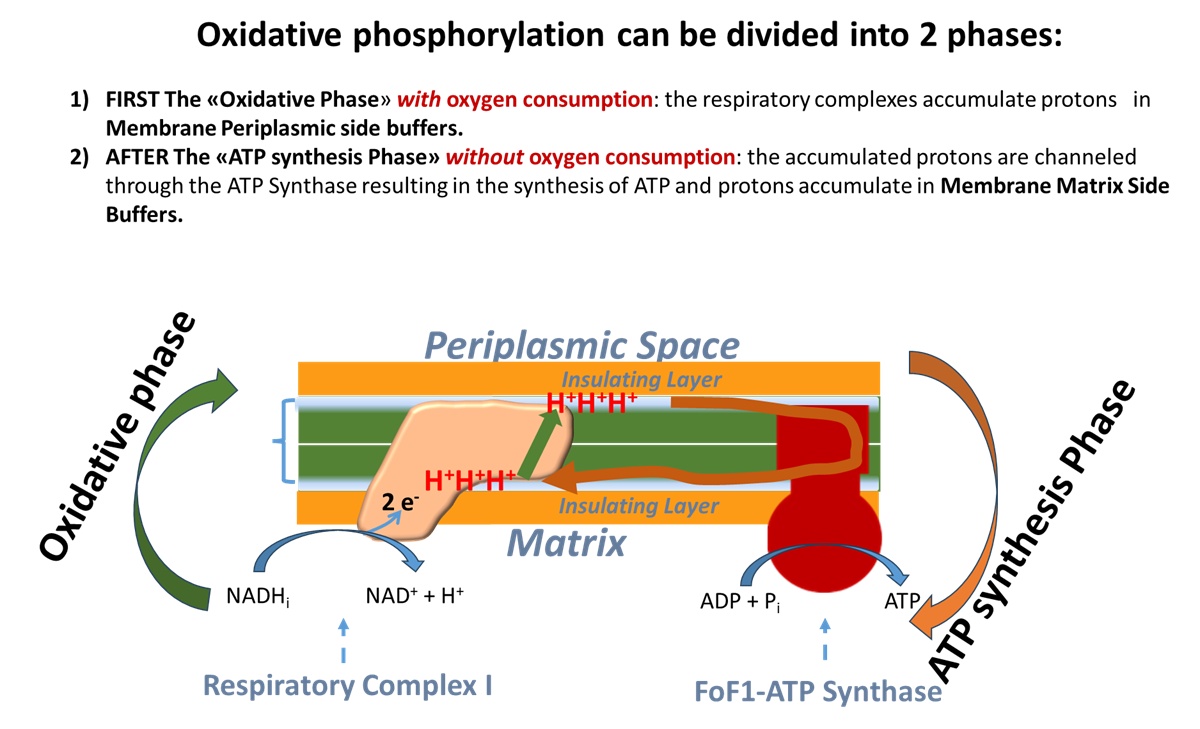
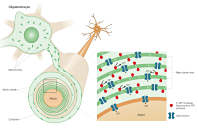
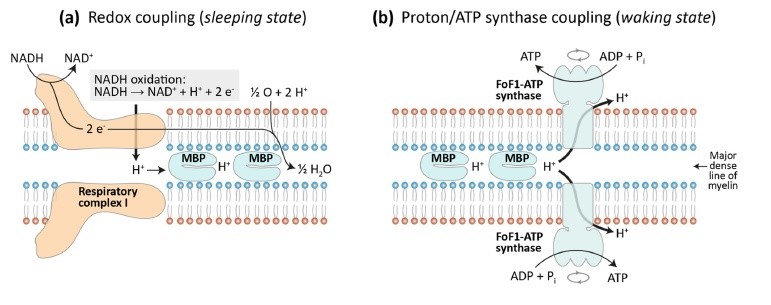
This slide depicts in green the first "Oxidative Phase" of oxidative phosphorylation, which occurs with an electron transport chain activated by NADH that unloads electrons onto oxygen, reducing it to H2O. Protons are transferred from Respiratory Complex I in the buffer on the matrix-facing membrane layer to the buffer on the periplasmic space-facing membrane layer.
In brown the slide depicts the proton "unloading" or "ATP synthesis phase" from the periplasmic buffer to the matrix buffer, passing through the Fo subunit of ATP synthase, resulting in ATP synthesis.
2025-11-26 Alessandro Maria Morelli gave an invited presentation at the Targeting Mitochondria conference, Berlin, October 22-24, 2025, entitled "Export of the Macromolecular Devices for Efficient ATP Synthesis in Extra-Mitochondrial Districts. A mitochondria-sleep-Covid-19 connection," which attracted considerable interest.
Above I report a summary slide of the presentation held in Berlin.
Abstract
Introduction: The outer segments of vertebrate photoreceptor rods, the platelets, the plasmacells, the myelin sheath have a higher ATP synthesis capacity than mitochondria. Therefore, there must be pathways that lead to the delocalization of the typically mitochondrial molecular machinery of oxidative phosphorylation (OXPHOS) and investigations have been carried out on this.
Results: The existence of both cellular and extracellular Mitochondria Derived Vesicles capable of synthesizing ATP is well documented. This appears to stem from the ease with which mitochondria produce vesicles, a property evidently inherited from the bacteria from which mitochondria derive, supporting the endosymbiotic theory. This process appears to work by allowing many cellular districts to locally synthesize ATP, the essential energy transfer compound that supports almost all intracellular processes.Thus, the hypothesis is gaining ground that mitochondria, with this vesiculation and membrane fusion, enable widespread extramitochondrial ATP synthesis. The myelin sheath surrounding the nerve presents a range of components of mitochondrial origin that appear to originate from mitochondria generated in the oligodendrocyte that fuse into the developing myelin. Laboratory tests detected the ability of myelin to store energy in the form of protons absorbed by the basic proteins - myelin basic protein and proteolipid - which myelin is rich in, and so the function of proton capacitor was attributed to myelin.
Perspectives: This ability has allowed us to formulate a theory about sleep that involves the accumulation of protons, and therefore energy, by myelin during sleep. This energy is converted into ATP during wakefulness to support energy-consuming nerve conduction. Sleep disturbances are common in many pathologies, such as COVID-19 infection, and this appears to be linked to the existence of amino acid sequence homology between the COVID-19 virus capsid and myelin proteolipid whereby Covid-19 infection produces antibodies against the proteolipid, unbalancing its function as a proton capacitor and consequently on sleep.
Link: https://wms-site.com/component/speakers/?view=speaker&id=11
2025-01-05 The article "Myelin: A possible proton capacitor for energy storage during sleep and energy supply during wakefulness" was published in Progress in Biophysical and Molecular Biology (link: www.doi.org/10.1016/j.pbiomolbio.2025.03.001 ). Authors: Alessandro Morelli, Ann Saada, Felix Scholkmann. Highlights:
- A new hypothesis as to why living organisms need sleep
- Sleep: accumulation of protons in the myelin
- Wakefulness: discharge of the myelin proton capacitor and production of ATP
2024-03-19 Alessandro Maria Morelli has published Lesson n° 3 of "Biochemical Updates " (Aggiornamenti di Biochimica). Title: "Mitochondria, Brain, Myelin - Permanent incorporation of the radioisotope 14C into human brain DNA coming from the 2418 nuclear tests of the years 1949-2009".
Link: https://www.youtube.com/watch?v=KFPSQgCnV8g
2024-02-26 Alessandro Maria Morelli published “Biochemistry Updates” on YouTube dedicated to exposing the background that led to his latest publication in the journal Biochimie.
Link: https://www.youtube.com/@AggiornamentidiBiochimic-sv8jq
2024-02-09 Alessandro Maria Morelli announces significant innovations in biochemistry:
1) Mitochondria possess the molecular machinery to synthesize ATP but cannot pour it into the cell because they are hindered by the double membrane system which hinders the exchange of incoming ADPà outgoing ATP.
2) Mitochondria have an intense vesiculating activity with which they export primarily to the endoplasmic reticulum (ER) the molecular machinery that carries out oxidative phosphorylation (OXPHOS) with the associated synthesis of ATP.
3) An active synthesis of ATP via OXPHOS occurs on the ER as illustrated by the article by Alessandro Maria Morelli & Felix Scholkmann published in open form on 31 January 2024 by the journal Biochimie.
Recent publication by Alessandro Maria Morelli & Felix Scholkmann:
Should the standard model of cellular energy metabolism be reconsidered? Possible coupling between the pentose phosphate pathway, glycolysis and extra-mitochondrial oxidative phosphorylation.
Biochimie. 2024 Jan 31:S0300-9084(24)00036-1. doi: 10.1016/j.biochi.2024.01.018. PMID: 38307246.
Link: https://www.sciencedirect.com/science/article/pii/S0300908424000361
Highlights:
-
Cellular respiration: still not fully understood and several anomalies discovered.
-
We present a new model of cellular energy metabolism.
-
Our model: glucose metabolism is coupled to the pentose phosphate pathway and extra-mitochondrial oxidative phosphorylation in a closed-loop process.
-
ATP production appears to be only a secondary function of the mitochondria.
2024-02-01News on mitochondria: can they be exclusive aerobic ATP producers?
We have recently reported data showing that the functionality of the aerobic ATP synthesis can even be more effective in subcellular districts other than mitochondria, such as rod outer segment disks and myelin sheath, thanks to the ectopic expression of the mitochondrial oxidative phosphorylation (OXPHOS) proteins. This fact will not affect in any way the importance of mitochondria. In fact these constitute a physiological niche where the assembly of the complex molecular system for the (OXPHOS) takes place.
Our recent observations demonstrate that mitochondrial ability to aerobically synthesize ATP is quite poor. This may seem heretical, but descends from experimental data.
The hypothesis emerges that this can be related to the intrinsic functioning of the system for the exchange of ADP and ATP across the two mitochondrial membranes.
Sensitivity of photoreceptors to degenerative processes
Our data published rod outer segment disks that suggests that the metabolic support to phototransduction in the rod outer segment (OS) may originate directly in the OS, able to conduct OXPHOS. This oxygen-handling activity of the rod OS, which was never suspected before, may be a primary source of reactive oxygen species in the OS.
Reactive oxygen species would generated by the ectopic mitochondrial electron transport chain (ETC) expressed in rod OS disks.
Knowledge of this fact may shed new light on the pathogenesis of those neurodegenerative retinal pathologies that are caused by oxidative stress, such as diabetic retinopathy, age-related macular degeneration, retinopathy of the prematurity, and photoreceptor cell death after retinal detachment.
Reassessment of the shunt pentose phosphate pathway
Recent literature data review suggests that the pentose-phosphate shunt would be pivotal for the catabolism of glucose, more than previously thought.
Serious doubt is cast to the stage catalyzed by aldolase in the first step of glycolysis, which it may suffer from a thermodynamic stop.
It can be envisaged that the pentose-phosphate shunt would be able to directly link hexose monophosphates to triose phosphates thus bypassing the aldolase blockage. Furthermore the pentose-phosphate shunt forms fructose-6-P which, thanks to phosphogluco-isomerase, can in turn resynthesizes glucose-6-P that can be sent again to the shunt reactions, thus implementing a highly versatile recycling that leads ultimately to the conversion of sugars to CO2 with consequent energy extraction.
This on a bioenergetic basis assures the cell a source of energy rapid and easily expendable. Therefore, the pentose-phosphate shunt would play both a catabolic and anabolic role.